FDA Safety Communication on Withdrawal Itch from Cetirizine and Levocetirizine

If your child uses or has been using the antihistamines cetirizine (Zyrtec) or levocetirizine (Xyzal) as a maintenance therapy, this one’s for you — and yes, this includes you grown‑ups too!
The U.S. Food & Drug Administration (FDA) recently issued a safety alert about a rather unexpected outcome: a withdrawal‑itch (aka pruritus) or rash after stopping long‑term use of these medications. That means one day your child stops taking the medicine, and days later, wakes up itchy. Not fun, but the good news is it’s very rare, and there are things you can do to manage it.
In this article, we’ll talk about the “why” and “how” of this phenomenon, give you practical tips as a parent, and guide you on when it’s time to ring up your pediatrician.
FDA Safety Communication and Its Purpose
The FDA’s formal announcement titled “FDA requires warning about rare but severe itching after stopping long‑term use of oral allergy medicines cetirizine or levocetirizine” came out in May 2025.
Here’s the key part: the FDA found that, in some patients who had been taking cetirizine or levocetirizine daily for months or years, stopping treatment led to severe itching (pruritus). The itching wasn’t present before the medicine. It appeared within a few days after stopping.
Why does the FDA issue safety communications like this? Because even if a side effect (or withdrawal effect) is rare, once it becomes noticeable and reportable, the risk‑vs‑benefit balance for long‑term use of that medicine may change for some people. The FDA’s role is to inform healthcare providers and patients, update labels if necessary, and encourage better reporting of adverse effects.
In this case, the FDA will require the prescribing information for prescription cetirizine and levocetirizine to include a new warning about pruritus after stopping long-term use. They will also ask over-the-counter (OTC) manufacturers to update their Drug Facts labels.
As a parent or caregiver, this isn’t meant to scare you into never starting or stopping an antihistamine. It’s simply to raise awareness: if you or your child has been on one of these agents long‑term, you should talk to your doctor before suddenly stopping the medication.
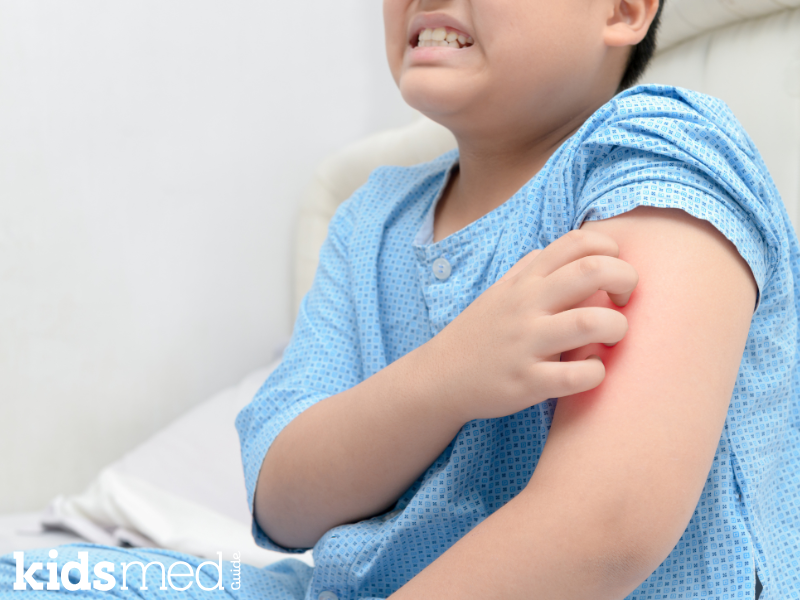
Why Withdrawal Itch and Rash Occur
Cetirizine withdrawal itch and levocetirizine withdrawal rash are phrases used to describe these symptoms after stopping the medication. The “itch” is the most prominently reported symptom; the rash may accompany or follow.
So why might this happen?
- Cetirizine and levocetirizine are second‑generation H1‑antihistamines. They block histamine receptors in the body (the ones that trigger itching, sneezing, and hives in allergies).
- When someone uses such an antihistamine daily for a long time, the body may adapt in subtle ways (e.g., receptor up‑regulation, changes in histamine‑release mechanisms, etc.).
- If the medicine is stopped abruptly after long‑term use, the “block” is removed — the histamine system may temporarily overreact before it settles back down. That rebound may manifest as widespread itching or rash.
- It’s worth noting that this phenomenon is rare: about 209 global cases reported between April 2017 and July 2023 for cetirizine and levocetirizine.
There has not been a formal, rigorous study to prove or disprove this association, or to explain why it occurs in some patients. So far, we have had just enough association for the FDA to alert the public and medical community.
Bottom line: for some individuals, the body may need a transition period when stopping long‑term therapy with cetirizine or levocetirizine.
How Can Parents and Patients Manage Symptoms Safely
Before stopping: plan ahead and talk with your healthcare provider
- If your child (or you) has been taking cetirizine or levocetirizine for a long time (months or years), talk with your pediatrician or pharmacist before stopping.
- The doctor may suggest tapering off (gradually reducing the dose) rather than stopping abruptly, especially if daily use has been extensive.
Check with your doctor whether long-term use of the antihistamine is necessary (for example, seasonal versus year-round needs or other ways to manage a medical condition).
If withdrawal itch occurs:
- Contact your healthcare provider for advice. Some withdrawal itch cases can be severe and need medical supervision.
- Your doctor may suggest restarting the antihistamine for a short period, then gradually weaning off it.
- Other medical treatments might be needed in severe cases, such as topical or oral steroids or other medications. Do this only under your pediatrician's guidance.
- Consider using gentle, fragrance-free moisturizers for dry or itchy skin, and applying cool compresses to itchy areas.
- Understand the timeline: Most cases noted that symptoms resolved when the medicine was restarted or when the person slowly tapered the dose.
After stopping, watch closely:
If your child has been on cetirizine or levocetirizine and you are stopping the medication, watch them closely. If you notice anything unusual, such as itching, swelling, or a rash, write it down (or take a picture) and contact your doctor. Mild itching may be managed at home, but watch it closely.
When to Seek Medical Attention
Itching or a rash after stopping an antihistamine is usually not an urgent emergency, but there are warning signs. Contact your pediatrician or healthcare provider immediately or seek emergency care if you notice:
- The itching is severe, preventing sleep or daily activities, and lasting more than a few days.
- A new rash develops that is spreading, blistering, and shows signs of infection (warmth, pus, swelling).
- Swelling of the face, lips, tongue, or throat (angioedema) or breathing difficulty — these are emergencies; call 911.
- Other symptoms such as fever, chills, joint pain, or any unusual systemic symptoms.

The Importance of Reporting Adverse Effects
If you notice significant itching or a rash after stopping cetirizine or levocetirizine, report it! Your healthcare provider can also help with this. You can also report any unknown or unusual side effect you believe may be due to a medication. The FDA gathers this information through its MedWatch program. As more data is collected, the FDA will investigate.
If sufficient information accumulates to suggest an association between a medication and a side effect, warnings will be issued, studies will be conducted, guidelines may change, and labels may be updated, benefiting everyone.
Conclusion
The FDA’s new safety communication highlights that cetirizine withdrawal itch and levocetirizine withdrawal rash can occur — in rare cases — when stopping these medications after long‑term daily use. The effect is usually seen within days.
If your child (or you) has been using these antihistamines long‑term, schedule a check‑in with your prescribing provider or pharmacist before stopping. If itching or rash arises, don’t brush it off or ignore it. Reach out to your medical provider.
Frequently Asked Questions
What is cetirizine withdrawal itch and levocetirizine withdrawal rash?
These terms refer to new or worsening itching (pruritus) or rash that has been reported when someone stops taking cetirizine or levocetirizine after long‑term daily use (months to years).
How long do withdrawal symptoms usually last?
Symptoms typically began within a few days of stopping. Some patients improved when they restarted the medicine or tapered gently.
Can children experience these symptoms?
Yes — although the published case series have more adults, children who have been on these medicines long‑term are potentially at the same risk.
Should I taper off antihistamines or stop them suddenly?
A taper may be a smart option in long‑term daily users of cetirizine or levocetirizine, rather than abrupt cessation, to reduce the chance of withdrawal itch or rash. Always consult your provider or pharmacist before making changes.
How can I report adverse effects to the FDA?
You or your child’s provider can use the FDA MedWatch form online (search “MedWatch FDA report a side effect”) or call the FDA’s MedWatch hotline to report new or worsening itching, rash, or other reactions after stopping cetirizine or levocetirizine.
The following references were used to compile this information:
Research, C. for D. E. and. (2025a). Drug Safety Communications. FDA. https://www.fda.gov/drugs/drug-safety-and-availability/drug-safety-communications
Research, C. for D. E. and. (2025b). FDA requires warning about rare but severe itching after stopping long-term use of oral allergy medicines cetirizine or levocetirizine (Zyrtec, Xyzal, and other trade names). FDA. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warning-about-rare-severe-itching-after-stopping-long-term-use-oral-allergy-medicines



